Background
Restoring a functional level of vision to people who are blind has been a dream of medicine for centuries. Although we are still far from restoration of high-resolution vision, bionic eye technologies are overcoming crucial bottlenecks and are slowly moving from the laboratory into the clinic and onto the free market.
History of Bionic Vision
Advances in medicine, surgery and electronics have set the stage for a fusion of the physical and biological sciences; one in which visual prostheses may restore lost functional capacity to the disabled. For excellent reviews on the subject, see [1, 2, 3].
The Early Days
The end of the 18th century saw the introduction of electrophysiology as a scientific discipline, opening the door to many studies of cortical stimulation.
1752
Benjamin Franklin theorized to the Royal Society of London that sight and hearing could be restored with the use of electricity [4].
1755
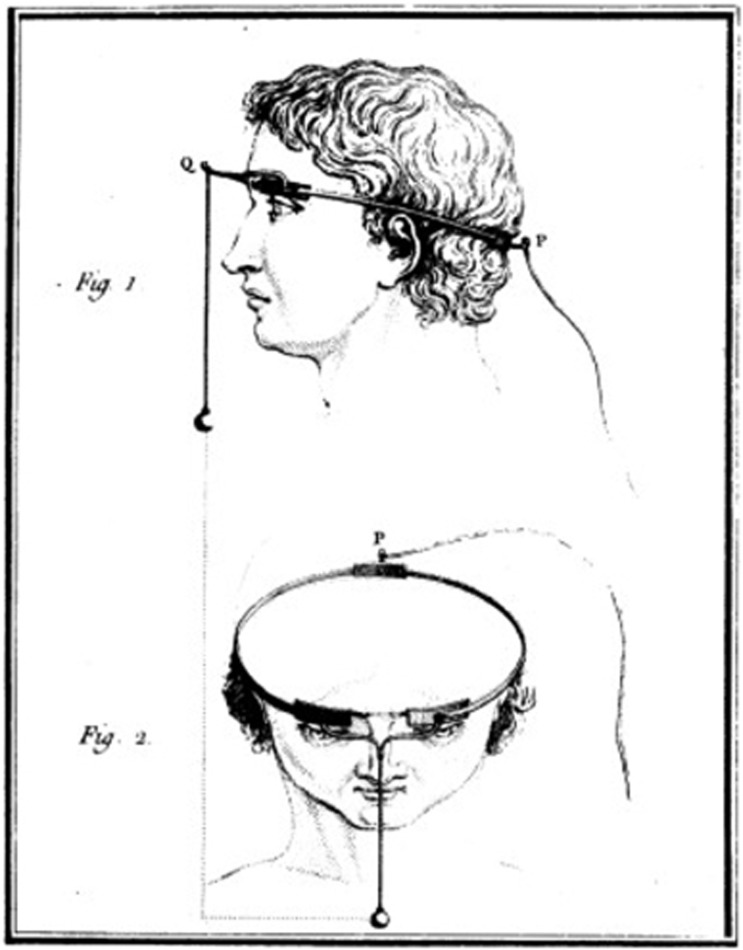
Charles Le Roy, an 18th century French scientist, conducted the first attempt to cure blindness through electrical stimulation (presumably after reading Benjamin Franklin's theory). Le Roy was able to evoke flashes of light (
phosphenes ) and other visual disturbances in a blind patient by sending electrical currents through a wire wrapped around the patient's head [5].Fig. 1: Method used by Charles Le Roy to evoke visual disturbances in a blind volunteer. A wire was wrapped around the patient's head with current applied through it. (reproduced with permission from [6])
1870
Two German physicists called Gustav Theodor Fritsch and Eduard Hitzig performed a number of experiments involving systemic stimulation across the cerebral hemispheres of dogs. These studies demonstrated that cerebral hemispheres were excitable at a time when many scientists believed that they were not [7].
1884
Victor Horsley, a British scientist, is credited as the first to use electrical stimulation of the
cerebral cortex intraoperatively. Horsley electrically stimulated an occipital encephalocele in a 6-week old child [8], in response to which the child's eyes showed rapid conjugate deviations.
Treatment of Head Injuries in the World War Era
The beginning of the 20th century saw the introduction of cortical stimulation as a treatment option for epilepsy. With the increased occurrence of gunshot and battlefield wounds to the head, neurosurgeons reported a wide variety of phosphenes in response to cortical stimulation.
1918
Kurt Löwenstein & Moritz Borchardt electrically stimulated the exposed visual cortex of a patient who suffered persistent seizures from a bullet wound on the left side of his head. The stimulation produced flickers of light that closely resembled the visual phenomena the patient would see preceding a seizure, showing that stimulation of the visual cortex could produce point sources of light [9].
1924
Fedor Krause introduced surgical operations for treatment of epilepsy in Germany, performing over 400 operations on epileptic patients. When he stimulated the cortex of a gunshot wound victim, the patient reported seeing jagged rings of light and stars in different patterns. Krause observed that even though his patient was
hemianopic for nine years, he still experienced visual hallucinations in his blind hemifield preceding a seizure. Electrical stimulation could provoke similar visual imagery in the blind hemifield as well [10].1929
Otfried Förster, a German neurologist and neurosurgeon, examined a patient with a projectile injury that resulted in occipital seizures. He demonstrated that stimulation of the
occipital pole and its surrounding cortex produced phosphenes that were motionless and located at the center of the patient’s visual field [2].1947
The American-Canadian neurosurgeon Wilder Graves Penfield examined 17 years of visual cortex stimulation in about 330 operations. He stimulated a wide area of the visual cortex and also one area outside the occipital lobe, which still produced visual imagery. The observations of Penfield were unique in that patients reported seeing phosphenes of different colors [11].
The First Generation of "Bionic Vision" Implants
By the 1950s, it was well known that electrical stimulation of the occipital poles could produce punctate phosphenes in the centre of the visual field [3]. This knowledge, plus Krause's observation that it was possible for a blind patient to perceive visual imagery upon stimulation of the occipital cortex, prompted the suggestion that a visual prosthesis might be a practical possibility.
1962
"I see a flash!" - Button (1958, p.54)
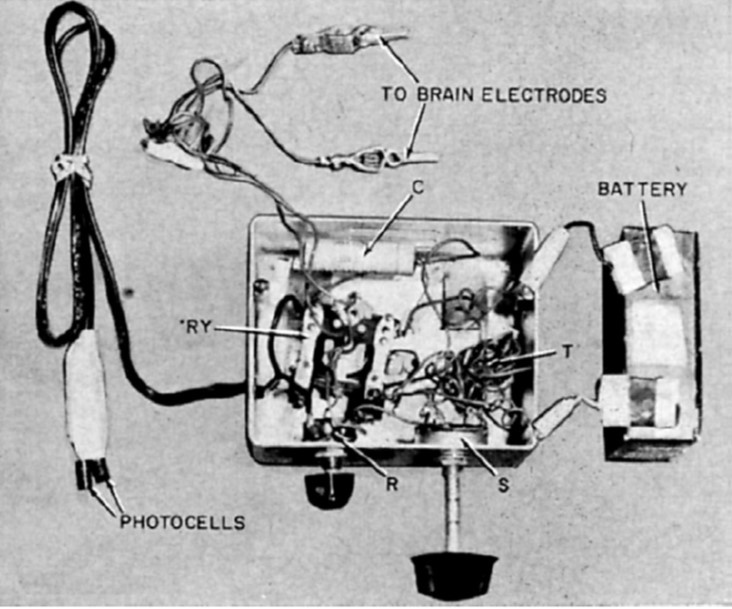
John C. Button and Tracey Putnam at Cedars of Lebanon Hospital (now Cedars-Sinai) in Los Angeles created and implanted the first ever bionic vision device in a 36-year old woman who had been completely blind for 18 years. Two pairs of stainless steel wires were implanted into the cortex and connected to a simple stimulator. The patient reported seeing vivid flashes of light and (with the use of a photocell) was able to detect the presence and relative brightness of a light source [3].
Fig. 2: Button's stimulating apparatus was connected to an array of four stainless steel wires implanted in the occipital cortex of a blind woman. (reproduced under CC BY-NC-ND 4.0 from [2])
1965
Giles Brindley, a physiologist at the University of Cambridge, had been undertaking a programme of research to support the development of a fully implanted, multielectrode wireless visual cortex stimulation device developed a fully implanted, multielectrode, wireless visual cortex stimulation device with the goal of allowing prosthesis recipients to read printed or handwritten text [5].
1966
The first Conference on Visual Prosthesis was held in Endicott House at the Massachusetts Institute of Technology. The conference chair Theodore Sterling explained that after initial difficulty in bringing together potential participants, the conference was finally held under the proviso that the proceedings would not be made publicly accessible [2].
1968
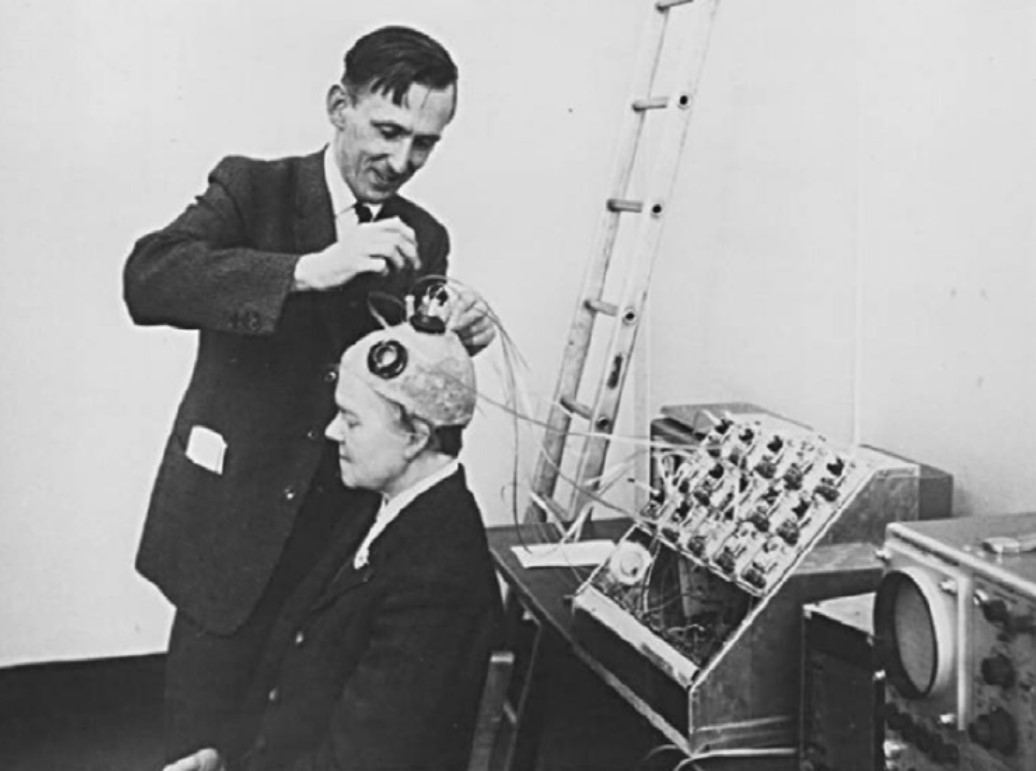
Brindley and Lewin implanted their first cortical implant that consisted of an array of 80 small, square, platinum electrodes embedded in a silicone cap. Each electrode was hard-wired to a receiving coil that was part of an array of coils also embedded in silicone and implanted under the scalp. 39 electrodes in the implant produced phosphenes across the patient’s visual field when stimulated. Centrally evoked phosphenes were small and flickering whereas peripherally evoked phosphenes differed more in size, shape, brightness, and sharpness [12].
Fig. 3: Brindley stimulating the visual cortex of his first implant recipient, placing a stimulating coil over a matched receiving coil implanted under her scalp. (reproduced under CC BY-NC-ND 4.0 from [2])
The Era of Government-Funded Research
The same year that Brindley & Lewin published their groundbreaking paper, the UK Medical Research Council formed the Neurological Prosthesis Unit, with Brindley as its director. In the US, the University of Utah instigated a sensory prosthesis research program with William Dobelle as director. In addition, the National Institute of Health (NIH) established a neuroprosthetic program at the US National Institute of Neurological Diseases and Blindness (NINDB, now known as NINDS). Thus began the era of government-funded research towards the development of a cortical visual prosthesis:
1969
The second Conference on Visual Prosthesis was held at the University of Chicago, aimed at trying to find future directions necessary to explore a possible visual prosthesis [2].
1974
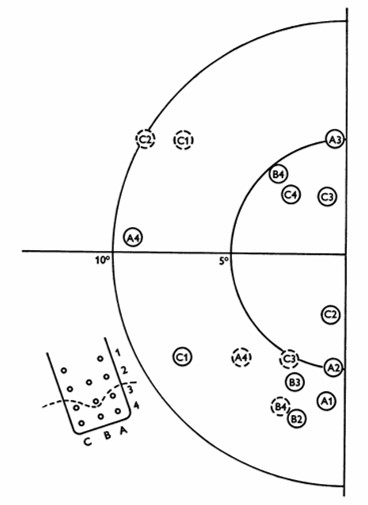
William Dobelle and his group developed a removable multielectrode cortical surface array, which was tested on 36 sighted patients undergoing occipital surgery [13] and 2 blind volunteers who had been blind for 7 and 28 years, respectively [14]. The array contained 64 hexagonally arranged platinum discs placed only over the mesial occipital cortex, each of 1-mm2 surface area. Their results in blind subject closely agreed with Brindley & Lewin's [12], noting that phosphenes could be elicited with stimulation of V2 and persisted with suprathreshold stimulation. The same was not true in sighted volunteers, where phosphenes did not always flicker and were occasionally coloured, always disappeared immediately upon cessation of stimulus, and gradually faded with continuous pulse trains [13].
Fig. 4: Phosphene map in the visual field for case no. 36. Phosphenes indicated by dashed circles appear only at high amplitudes. The electrode array and numbering system are also shown, along with a dashed line showing the postulated position of the calcarine fissure. (reproduced with permission from [14]).
1974
Over the same period, researchers from the Huntington Institute of Applied Medical Research also developed and tested a cortical array comprising smaller, "strip" arrays of 18 platinum surface electrodes, placed separately on both the occipital pole and mesial occipital cortex of normally sighted patients [2, 15]. The implant was able to elicit phosphenes, but could also cause seizures, which led the NIH to disbandon the group's effort in favor of research on "safe and effective methods for neural stimulation" [2].
1975
Another group funded by the NIH and based at the Massachusetts General Hospital in Boston had been undertaking intraoperative visual cortex stimulations on five patients throughout the early 1970s. However, only three patients reported seeing phosphenes, with the two failures attributed to postoperative swelling and patient drowsiness [2].
1978
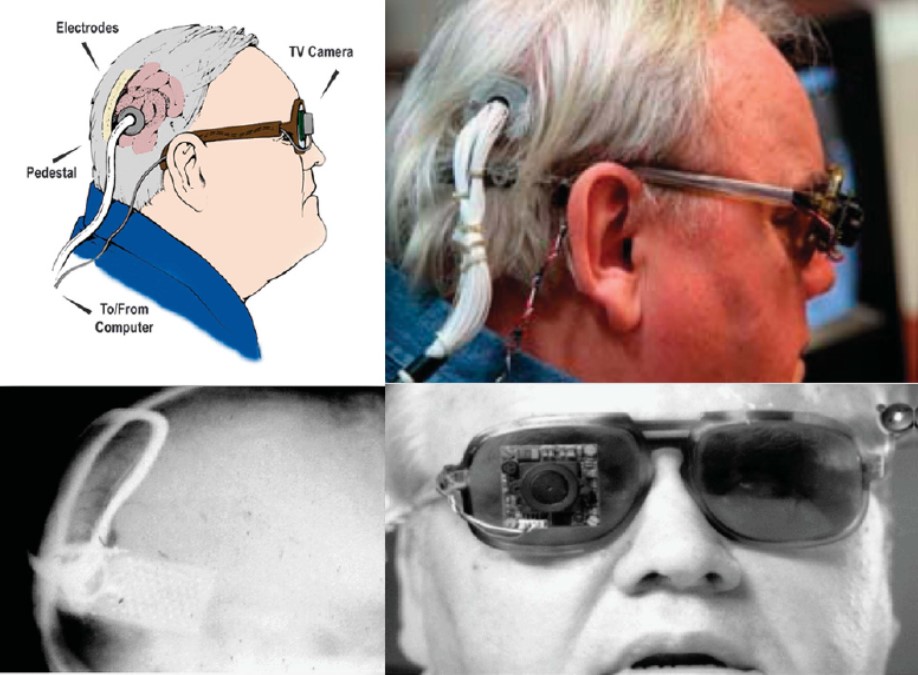
Dobelle and colleagues continued to improve their implant design throughout the 1970s, adding a transcutaneous connector to permit chronic implantation of electrodes and spectacle-mounted cameras. One of Dobelle's early patients retained his implant for over 20 years, with no reported complications of infection or seizure [2].
Fig. 5: Different views of a recipient of the Dobelle implant, first implanted in 1978. In 2000, his external hardware & software was upgraded, including the miniature camera shown, greatly enhancing the functionality of his prosthesis. (reproduced with permission from [6])
1982
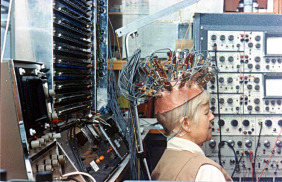
Determined to pursue the goal of reading conventional letterforms, Brindley's group continued developing improved implants into the 1980s including one with 151 electrodes [13], which unfortunately became infected and had to be removed [14].
Fig. 6: "Miss Bonnett", recipient of the last visual cortex implant built by the MRC Neurological Prostheses Unit. (reproduced under CC BY-NC-ND 4.0 from [2])
The Era of Retinal Implants
The considerable risk associated with cortical prostheses led to the scientific community to explore other locations along the visual pathway capable of producing phosphenes, which led to a new era focused on the development of retinal prostheses.
1996
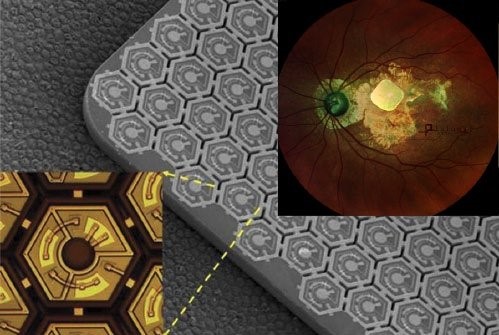
Mark Humayun and colleagues showed that local electrical stimulation of the retinal surface in patients with outer retinal diseases resulted in focal light perception, even though the patients had been blind for many years [16].
2011
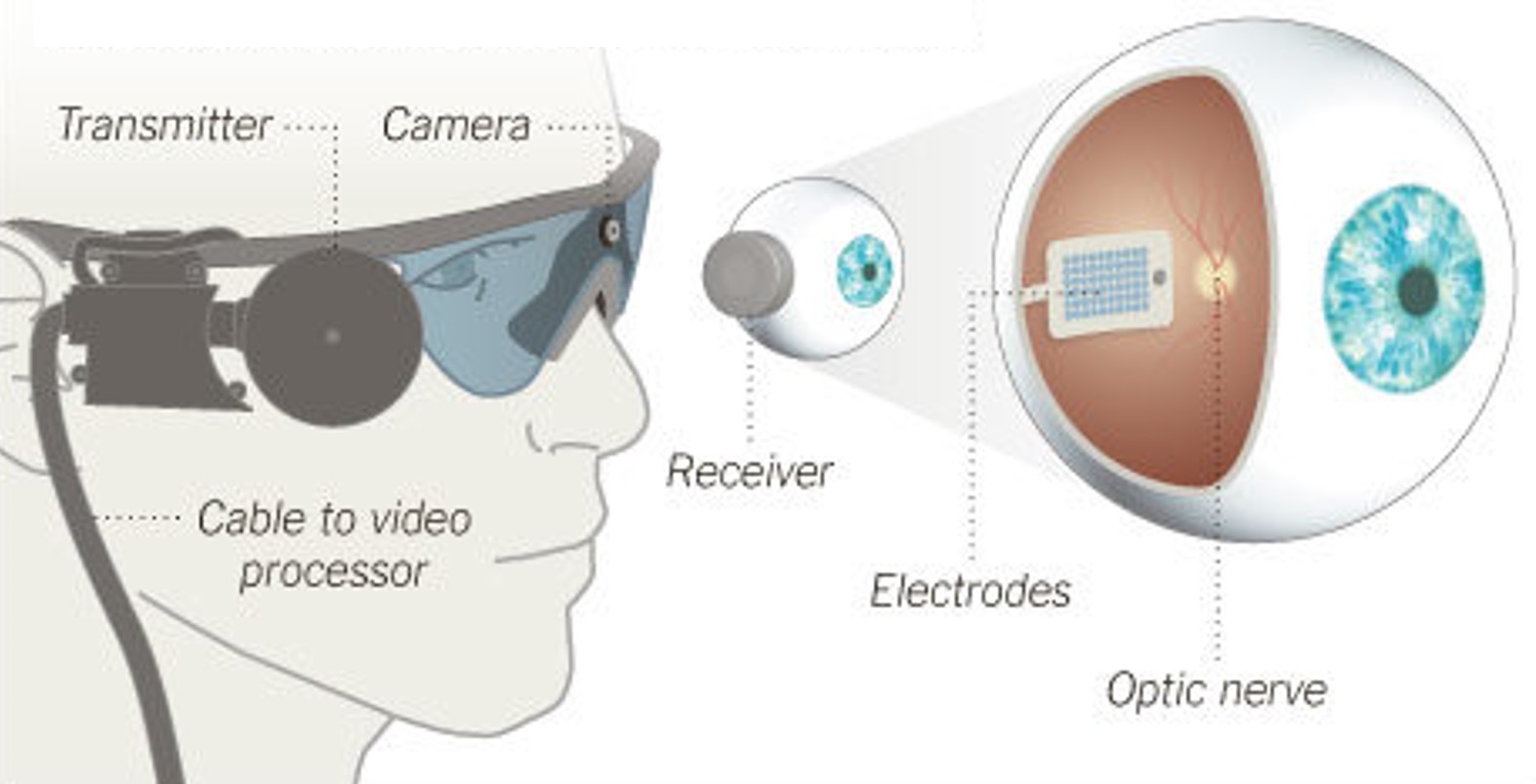
Argus II, an
epiretinal implant developed by Second Sight Medical Products, received approval for commercial use in the European Union (CE Mark). The implant was initially available at a limited number of clinics in Switzerland, France, and the United Kingdom, at an EU market price of $115,000 [17].Fig. 7: Argus II Retinal Prosthesis System (Source: Second Sight). Camera images are processed and transferred to electrodes implanted in the back of the eye.
2013

Alpha IMS, a
subretinal implant developed by Retina Implant AG, received the CE Mark as well [18]. The implant was initially available at around $130,000.Meanwhile in the US, Argus II became the first retinal implant to receive FDA approval [19].
Fig. 8: Alpha IMS, a subretinal implant consisting of a 3x3mm2 microchip with 1,500 electrodes (Source: Retina Implant AG).
2019
Retina Implant AG discontinued business activities quoting innovation-hostile climate of Europe's rigid regulatory and unsatisfactory results in patients.
2020
Stricken by fundraising issues due to COVID-19, Second Sight announced massive lay-offs and an intent to wind down operations. The company would later attempt a (failed) business merger with Pixium Vision but ultimately rebound after raising ~$60M in a public offering.
2021
Pixium Vision implanted their first of an expected 38
AMD patients with PRIMA, a subretinal implant, as part of their PRIMAvera clinical trial. This study is the last step before seeking market approval for dry AMD in Europe.In addition, Pixium expects to report 36-month data from the French feasibility study by early 2022, and is continuing clinical development in the US.
Fig. 9: PRIMA, a subretinal implant consisting of ~400 photovoltaic cells (Source: Pixium Vision).
The Future
2020s - ?
With dozens of next-generation implants in development that include retinal, optic nerve, and cortical approaches, a wide variety of bionic eye technologies should be available within a decade.
Causes of Vision Loss
Vision loss can occur in a number of ways. The main focus of retinal prosthetic research is to improve functional vision for people with blindness and/or vision impairment from the result of hereditary diseases, such as retinitis pigmentosa (RP) and age-related macular degeneration (AMD).
Disclaimer: The following is for informational purposes only. If you think you are subject to one of the conditions described below, please consult with your care provider.
Retinitis Pigmentosa
Retinitis pigmentosa (RP) is the most common form of inherited blindness that affects 1 in every 3000 – 7000. RP is the result of a change in a gene or genes that would typically lead to functioning photoreceptors, but instead create smaller photoreceptors with a disorderly arrangement. RP develops over a longer period of time compared to other vision diseases, sometimes spanning a person's entire life. Symptoms for RP usually include peripheral vision field loss (also known as "tunnel vision") and night blindness. Currently, there is no known cure for RP.
There are two classifications of RP: syndromic and non-syndromic. Non-syndromic RP is more common than syndromic RP and does not develop with other diseases or disorders. Syndromic RP can occur with other medical conditions or disorders where neurosensory systems other than the eye are affected. People with syndromic RP make up an estimated 20-30% of all RP patients. All RP types are a degradation of the retina caused by genetics, also known as a dystrophy. The rod-cone dystrophy is usually syndromic and involves a loss of cone activation due to relative low rod activation, which present with loss of central vision as well as the usual peripheral vision field loss associated with RP.
RP is associated with a number of different conditions. Usher syndrome, for example, is a combination of RP and deafness and is estimated to contribute to 14% of all RP cases overall. Bardet-Biedl syndrome is a rare genetic disorder that presents with rod-cone dystrophy. Some forms of RP are associated with mitochondrial disorders, such as the Kearns-Sayre syndrome, where the area of the macula is more affected than the periphery.
Implants that may be able to treat RP include Argus II, the suprachoroidal implant, and other retinal implants as well as cortical implants.
Age-Related Macular Degeneration (AMD)
In 2010, there were 2.07 million people with AMD. That number is expected to double to 5.44 million by 2050. As with most health conditions, aging is a significant risk factor for AMD.
The macula is a pigmented circular area located on the back of the eye on the retina. Within the macula is the fovea, an area responsible for processing our central field of view.
There are three factors that are used to characterize the different types of AMD: size of drusen, type of damage caused by progression, and presenting as either “wet” or “dry”. Small yellow deposits of drusen (made of proteins and lipids) can build up in the macula, usually as a sign of Early AMD. Intermediate AMD involves larger size drusen deposits and a change in retinal pigment functioning. While intermediate AMD can cause some vision loss, both early and intermediate AMD are typically present without other symptoms.
Late AMD presents with loss of central vision due to progressive damage to the retina, either as wet AMD or geographic atrophy. Wet AMD, also known as neovascular AMD, is specifically caused by irregular blood vessel growth. These blood vessels can be easily damaged allowing for blood and proteins to spread into the area of the macula. Damage that leads to the degradation of three specific areas of the eye (neighboring photoreceptors, retinal pigment epithelium, and the choriocapillaris) is referred to as geographic atrophy. Further research into how AMD can affect the different layers of the retina is needed to better understand the physiology of geographic atrophy.
Dry AMD includes any type of AMD that is not neovascular or wet. Dry AMD has a significantly higher prevalence of all AMD patients (estimated at 80% or more) compared to Wet AMD.
Implants that may be able to treat AMD include PRIMA as well as other retinal and cortical implants.
Sight Restoration Technologies
Bionic Vision is not limited to computer interfaces but instead encompasses a wide range of vision restoration and/or rehabilitation techniques.
Electronic Neuroprostheses
Neuroprostheses, better known as "implants", have been a common focus of research and investments into curing blindness. These implants come in the form of a miniature chip which is placed into a specific location of the retina (
Retinal implants function to replace damaged photoreceptor cell activation. However, if the disease has significantly interrupted the early visual pathway (i.e. the retina, optic nerve, or lateral geniculate nucleus), cortical implants may be the only option.
Optogenetic Neuroprostheses
Optogenetics is a technique that uses a combination of optical and genetic methods to restore vision loss. Once light-sensitive proteins are introduced to the area of the damaged photoreceptors, retinal neurons can be stimulated by using a specific range of light intensities and wavelengths.
Two types of optogenetic strategies have been studied: ion channel therapies and G-protein coupled receptor (GPCRs) therapies. Ion channel therapy research focuses on improving temporal sensitivity for vision. While slower than ion channels, GPCR therapies have demonstrated much promise for vision restoration given their higher sensitivity to light activation. Both have been used to study and treat retinal dystrophies and degenerative diseases.
There are a few companies in the process of developing accurate optogenetic studies for future use. The GenSight PIONEER preliminary trial combines optogenetic therapy with biomimetic goggles. These specially designed goggles help to compensate for spatiotemporally related visual distortions associated with optogenetic treatment.
Gene-Based Therapies
When there is a mutation of a gene, the synthesis of proteins that follows is affected such that there can be an increase or inhibition of function for that gene. Inhibiting the function of a protein within the retina can lead to diseases that cause blindness. Gene therapy and gene editing are two current genetic approaches being studied to prevent the spread of retinal disease and possibly restore vision.
Gene therapy involves using recombinant DNA to encode the desired DNA into a plasmid which is delivered into the cell by a vector. This is meant to cause the expression of the desired gene throughout the retina, and restore cellular function. A limitation of gene therapy is that it can only function to supplement the expression of a gene, not eliminate or suppress the desired genetic expression. There has been significant progress in gene therapy for treating Leber congenital amaurosis, which shows promise in treating more frequent diseases in vision such as AMD and RP.
Cell Therapy
Once they are successfully injected or transplanted into the retina, stem cells have the ability to form all cell types necessary for retinal regeneration. There are two types of stem cells that can do this: embryonic stem cells (ESCs) and induced pluripotent stem cells (iPSCs). ESCs and iPSCs can mature into pluripotent stem cells, and pluripotent cells are then able to differentiate into bipolar or photoreceptor cells.
Inserting stem cells within the retina may be conducted through an injection process or a transplantation. There have been multiple trials over the past ten years testing the usefulness of stem cell therapy within the retina, mostly yielding promising results. Research has focused on studying the effectiveness of stem cell therapy in creating retinal pigment epithelium (RPE) cells and photoreceptor cells. Data suggest that iPSC transplants can produce RPE cells and have potential to restore vision or even slow the progression of disease for AMD patients. There are currently no clinical trials that have demonstrated the effectiveness of stem cell therapy for photoreceptor cells.
Sensory Substitution
Sensory substitution devices change visual information to auditory or tactile information. What is most notable about these solutions is that they do not require invasive surgery for use, instead relying on the plasticity of other sensory modalities to adapt new strategies to compensate for vision loss. When paired with extensive training, people who are blind or have low vision can improve performance in navigation, object recognition, and more.
Implants in this category include the vOICE (visual to auditory) and BrainPort (visual to tactile).
Glossary
Age-related macular degeneration (AMD or ARMD)
Macular degeneration, also known as age-related macular degeneration (AMD or ARMD), is a medical condition which may result in blurred or no vision in the center of the visual field. Early on there are often no symptoms. Over time, however, some people experience a gradual worsening of vision that may affect one or both eyes. While it does not result in complete blindness, loss of central vision can make it hard to recognize faces, drive, read, or perform other activities of daily life.
Cataract
A cataract is a cloudy area in the lens of the eye that leads to a decrease in vision. Cataracts often develop slowly and can affect one or both eyes. Symptoms may include faded colors, blurry or double vision, halos around light, trouble with bright lights, and trouble seeing at night. This may result in trouble driving, reading, or recognizing faces. Poor vision caused by cataracts may also result in an increased risk of falling and depression. Cataracts cause half of all cases of blindness and 33% of visual impairment worldwide.
Cerebral cortex
The cerebral cortex, also known as the cerebral mantle, is the outer layer of neural tissue of the cerebrum of the brain in humans and other mammals. The cerebral cortex mostly consists of the six-layered neocortex. It is separated by two cortices, by the longitudinal fissure that divides the cerebrum into the left and right cerebral hemispheres. The cerebral cortex is the largest site of neural integration in the central nervous system. It plays a key role in attention, perception, awareness, thought, memory, language, and consciousness.
Clinical trial
Clinical trials are experiments or observations done in clinical research. Such prospective biomedical or behavioral research studies on human participants are designed to answer specific questions about biomedical or behavioral interventions, including new treatments and known interventions that warrant further study and comparison. Clinical trials generate data on dosage, safety and efficacy. They are conducted only after they have received health authority/ethics committee approval in the country where approval of the therapy is sought.
Congenitally blind
If an infant is born unable to see or with severe visual impairment, he is said to have congenital blindness. A number of different conditions can cause congenital blindness, including certain diseases and genetic factors. The term “congenital” simply means that it is present from birth, and does not provide information as to why a child might be born blind. These patients have reduced acuity, severe nearsightedness and involuntary movements of the eyes.
Cortical prosthesis
A cortical implant is a subset of neuroprosthetics that is in direct connection with the cerebral cortex of the brain. By directly interfacing with different regions of the cortex, the cortical implant can provide stimulation to an immediate area and provide different benefits, depending on its design and placement. A typical cortical implant is an implantable micro electrode array, which is a small device through which a neural signal can be received or transmitted. The goal of a cortical implant and neuroprosthetic in general is “to replace neural circuitry in the brain that no longer functions appropriately.”
Diabetic retinopathy
Diabetic retinopathy, also known as diabetic eye disease (DED), is a medical condition in which damage occurs to the retina due to diabetes mellitus. Poorly controlled blood sugar is a risk factor and early symptoms include floaters, blurriness, dark areas of vision, and difficulty perceiving colors. It is a leading cause of blindness in many developed countries. Diabetic retinopathy affects up to 80% of those who have had diabetes for 20 years or more. At least 90% of new cases could be reduced with proper treatment and monitoring of the eyes. The longer a person has diabetes, the higher his or her chances of developing diabetic retinopathy. Each year in the United States, diabetic retinopathy accounts for 12% of all new cases of blindness.
Electrophysiology
Electrophysiology is the branch of physiology that studies the electrical properties of biological cells and tissues. It involves measurements of voltage changes, electrical currents, or manipulations on a wide variety of scales from single ion channel proteins to whole organs like the heart. In neuroscience, it includes measurements of the electrical activity of neurons, and, in particular, action potential activity. Recordings of large-scale electrical signals from the nervous system, such as electroencephalography, may also be referred to as electrophysiological recordings. They are useful for electro diagnosis and monitoring.
Epiretinal implant
Epiretinal implants are placed on top of the retinal surface, above the nerve fiber layer, directly stimulating ganglion cells and bypassing all other retinal layers. Array of electrodes are stabilized on the retina using micro tacks which penetrate into the sclera.
Glaucoma
Glaucoma is a group of eye diseases which result in damage to the optic nerve (or retina) and cause vision loss. The most common type is open-angle glaucoma, in which the drainage angle for fluid within the eye remains open, with less common types including closed-angle glaucoma and normal-tension glaucoma. Open-angle glaucoma develops slowly over time and there is no pain. Closed-angle glaucoma can present gradually or suddenly. Vision loss from glaucoma, once it has occurred, is permanent. Risk factors for glaucoma include increasing age, and use of steroid medication. If treated early, it is possible to slow or stop the progression of disease with medication, laser treatment, or surgery. The goal of these treatments is to decrease eye pressure.
Hemianopia
Hemianopia, sometimes called hemianopsia, is partial blindness or a loss of sight in half of your visual field. It's caused by brain damage, rather than a problem with your eyes.
Intracortical implant
Late blind
Late blindness refers to individuals who developed blindness at a later stage in life, after childhood. Late blindness as compared to early blindness may have the disadvantage of not having as much time to adjust and heighten connections of the sight, hearing, and touch centers of the brain to make up for vision loss.
Lateral geniculate nucleus (LGN)
The lateral geniculate nucleus (LGN) is a relay center in the thalamus for the visual pathway. It receives visual information from the retina and sends it to the visual cortex for processing. It is a small, ovoid, ventral projection of the thalamus where the thalamus connects with the optic nerve. There are two LGNs, one on the left and another on the right side of the thalamus. The LGN receives information directly from the ascending retinal ganglion cells via the optic tract and from the reticular activating system. Neurons of the LGN send their axons through the optic radiation, a direct pathway to the primary visual cortex. In addition, the LGN receives many strong feedback connections from the primary visual cortex. In humans as well as other mammals, the two strongest pathways linking the eye to the brain are those projecting to the dorsal part of the LGN in the thalamus, and the superior colliculus. .
Legally blind
Legal blindness is when vision is 20/200 or less in your better eye or your field of vision is less than 20 degrees. The American government uses the term “legal blindness” to decide who can get certain benefits, like disability or job training.
Macular degeneration
Occipital lobe
The occipital lobe is one of the four major lobes of the cerebral cortex in the brain of mammals. It is located at the rear end of the brain and is the visual processing center of the mammalian brain containing most of the anatomical region of the visual cortex. The occipital lobe is divided into several functional visual areas. Each visual area contains a full map of the visual world. Although there are no anatomical markers distinguishing these areas, physiologists have used electrode recordings to divide the cortex into different functional regions.
Optic nerve
The optic nerve is a paired cranial nerve that transmits visual information from the retina to the brain. It connects the eye to the brain and transmits all visual information including brightness perception, color perception and visual acuity. It extends from the optic disc to the optic charisma and continues as the optic tract to the lateral geniculate nucleus, pretectal nuclei, and superior colliculus. In most mammals, optic nerve damage results in irreversible blindness. The fibers from the retina run along the optic nerve to nine primary visual nuclei in the brain, from which a major relay inputs into the primary visual cortex. The optic nerve is composed of retinal ganglion cell axons and glia.
Optogenetics
Optogenetics is a biological technique that involves the use of light to control neurons that have been genetically modified to express light-sensitive ion channels. As such, optogenetics is a neuromodulation method that uses a combination of techniques from optics and genetics to control the activities of individual neurons in living tissue- even within freely-moving animals.
Partially sighted
Partially sighted indicates some type of visual problem that may require special education services such as readers, audio taped texts, and raised-line drawings. A partially blind individual may meet the challlenge of disability in the same way as a totally blind individual.
Phosphene
A phosphene is the phenomenon of seeing light without light actually entering the eye. Many patients have reported phosphenes as bright circular or streaky spots of light among darkness. Phosphenes can be directly induced by mechanical, electrical, or magnetic stimulation of the retina or visual cortex, or by random firing of cells in the visual system. Visual neuroprostheses have been found to stimulate phosphenes to restore vision to people with severe visual impairments. Most vision researchers believe that phosphenes result from the normal activity of the visual system after stimulation of one of its parts from some stimulus other than light.
Primary visual cortex (V1)
The primary visual cortex, also known as V1, Brodmann area 17, or the striate cortex, is the most studied visual area in the brain. It is a structure that is essential for conscious processing of visual stimuli. In mammals, it is located in the posterior pole of the occipital lobe and is the simplest, earliest cortical visual area. Sensory input originating from the eyes travels through the lateral geniculate nucleus in the thalamus and then reaches the visual cortex. It is highly specialized for processing information about static and moving objects, among other things.
Retinal ganglion cell
A retinal ganglion cell (RGC) is a type of neuron located near the inner surface (the ganglion cell layer) of the retina of the eye. It receives visual information from photoreceptors via two intermediate neuron types: bipolar cells and amacrine cells. RGC’s collectively transmit image-forming and non-image forming visual information from the retina in the form of action potential to several regions in the thalamus, hypothalamus, and mesencephalon. RGCs vary significantly in terms of their size, connections, and responses to visual stimulation but they all share the defining property of having a long axon that extends into the brain. These axons form the optic nerve, optic chaism, and optic tract.
Retinal implant
Retinal prosthesis
Retinal prostheses, a type of bionic eye, are implantable electronic devices designed to stimulate sensation of vision in the eyes of individuals with significant retinal diseases such as retinitis pigmentosa or age-related macular degeneration, where the optic nerve and visual cortex are unaffected. restoration of vision may be achieved by creating devices, retinal prostheses, that receive and process incoming light and then transmit the information in the form of electrical impulses to the remaining inner retinal layers for visual function. Although visual prostheses may conceivably be developed for any part of the visual pathway including the visual cortex itself, the retina is considered to be the easiest target for treating outer retinal disease due to its easy access and partially intact geospatial neuronal architecture and processing units. Designs for retinal prostheses are generally categorized based on where in the eye the device is intended to be placed and how the device delivers electrical impulses to RGCs.
Retinitis pigmentosa (RP)
Retinitis pigmentosa (RP) is a genetic disorder of the eyes that causes loss of vision. It is estimated to affect 1 in 4,000 people and is generally inherited from a person’s parents. Symptoms include trouble seeing at night and decreased peripheral vision. Onset of symptoms is generally gradual and often in childhood. The underlying mechanism involves the progressive loss of rod photoreceptor cells in the back of the eye which is generally followed by loss of cone photoreceptor cells. There is currently no cure for RP.
Secondary visual cortex (V2)
The secondary visual cortex, also known as V2, or prestriate cortex, is the second major area in the visual cortex, and the first region within the visual association area. It receives strong feedforward connections from V1 and sends strong connections to V3, V4, and V5.
Subretinal implant
Subretinal implants sit on the outer surface of the retina, between the photoreceptor layer and the retinal pigment epithelium, directly stimulating retinal cells and relying on the normal processing of the inner and middle retinal layers. Adhering a sub retinal implant in place is relatively simple, and consists of a silicon wafer containing light sensitive microphotodiodes, which generate signals directly from the incoming light.
Suprachoroidal implant
Suprachoroidal implants are placed between the posterior blood supply of the eye (choroid) and the outer white layer of the eye (sclera), with this surgical location primarily being chosen for stability and safety
Totally blind
Total blindness describes individuals with eye disorders who have no light perception. That is, a person who’s totally blind doesn’t see any light at all. Total blindness can be the result of trauma, injury, or various conditions.
Visual acuity
Visual acuity is a measurement determined by the letter chart tests we take when we get our eyes checked; the number represents your eyes’ clarity or sharpness. For example, a person with a visual acuity measurement of 20/70 who is 20 feet away from an eye chart sees what a person with 20/20 vision can see from 70 feet away.
Visual field
The entire area that can be seen when viewing a single point. Visual field tests are used to determine how a disease has affected loss of vision.
A
C
D
E
G
H
I
L
M
O
P
R
S
T
V
Quick Links
Contact Us
This is a community project. Find out more about our team of contributors. If you'd like to contribute, please contact us.
Media Copyright Information
All images used with permission. Introduction graphic: © Getty. Background graphic: © Tracy Spohn/Shutterstock.
Made with at UC Santa Barbara, with generous support from the bionic vision community (2020—2026).
 All our content (unless otherwise noted) is licensed under CC-BY 4.0.
All our content (unless otherwise noted) is licensed under CC-BY 4.0.