Background
Restoring a functional level of vision to people who are blind has been a dream of medicine for centuries. Although we are still far from restoration of high-resolution vision, bionic eye technologies are overcoming crucial bottlenecks and are slowly moving from the laboratory into the clinic and onto the free market.
History of Bionic Vision
Advances in medicine, surgery and electronics have set the stage for a fusion of the physical and biological sciences; one in which visual prostheses may restore lost functional capacity to the disabled. For excellent reviews on the subject, see [1, 2, 3].
The Early Days
The end of the 18th century saw the introduction of electrophysiology as a scientific discipline, opening the door to many studies of cortical stimulation.
1752
Benjamin Franklin theorized to the Royal Society of London that sight and hearing could be restored with the use of electricity [4].
1755
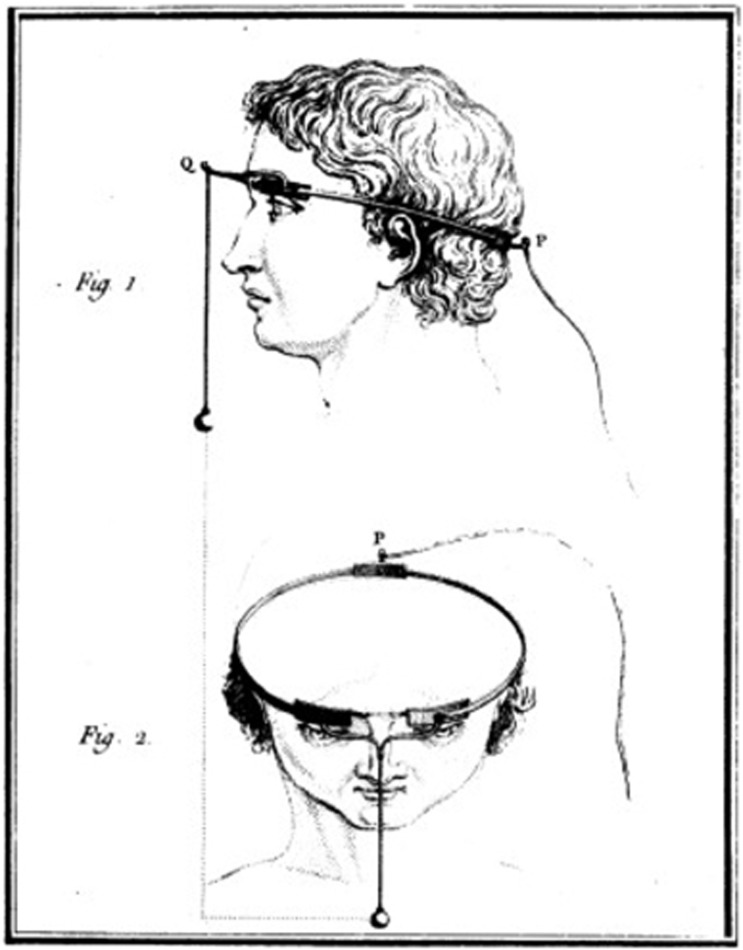
Charles Le Roy, an 18th century French scientist, conducted the first attempt to cure blindness through electrical stimulation (presumably after reading Benjamin Franklin's theory). Le Roy was able to evoke flashes of light (
phosphenes ) and other visual disturbances in a blind patient by sending electrical currents through a wire wrapped around the patient's head [5].Fig. 1: Method used by Charles Le Roy to evoke visual disturbances in a blind volunteer. A wire was wrapped around the patient's head with current applied through it. (reproduced with permission from [6])
1870
Two German physicists called Gustav Theodor Fritsch and Eduard Hitzig performed a number of experiments involving systemic stimulation across the cerebral hemispheres of dogs. These studies demonstrated that cerebral hemispheres were excitable at a time when many scientists believed that they were not [7].
1884
Victor Horsley, a British scientist, is credited as the first to use electrical stimulation of the
cerebral cortex intraoperatively. Horsley electrically stimulated an occipital encephalocele in a 6-week old child [8], in response to which the child's eyes showed rapid conjugate deviations.
Treatment of Head Injuries in the World War Era
The beginning of the 20th century saw the introduction of cortical stimulation as a treatment option for epilepsy. With the increased occurrence of gunshot and battlefield wounds to the head, neurosurgeons reported a wide variety of phosphenes in response to cortical stimulation.
1918
Kurt Löwenstein & Moritz Borchardt electrically stimulated the exposed visual cortex of a patient who suffered persistent seizures from a bullet wound on the left side of his head. The stimulation produced flickers of light that closely resembled the visual phenomena the patient would see preceding a seizure, showing that stimulation of the visual cortex could produce point sources of light [9].
1924
Fedor Krause introduced surgical operations for treatment of epilepsy in Germany, performing over 400 operations on epileptic patients. When he stimulated the cortex of a gunshot wound victim, the patient reported seeing jagged rings of light and stars in different patterns. Krause observed that even though his patient was
hemianopic for nine years, he still experienced visual hallucinations in his blind hemifield preceding a seizure. Electrical stimulation could provoke similar visual imagery in the blind hemifield as well [10].1929
Otfried Förster, a German neurologist and neurosurgeon, examined a patient with a projectile injury that resulted in occipital seizures. He demonstrated that stimulation of the
occipital pole and its surrounding cortex produced phosphenes that were motionless and located at the center of the patient’s visual field [2].1947
The American-Canadian neurosurgeon Wilder Graves Penfield examined 17 years of visual cortex stimulation in about 330 operations. He stimulated a wide area of the visual cortex and also one area outside the occipital lobe, which still produced visual imagery. The observations of Penfield were unique in that patients reported seeing phosphenes of different colors [11].
The First Generation of "Bionic Vision" Implants
By the 1950s, it was well known that electrical stimulation of the occipital poles could produce punctate phosphenes in the centre of the visual field [3]. This knowledge, plus Krause's observation that it was possible for a blind patient to perceive visual imagery upon stimulation of the occipital cortex, prompted the suggestion that a visual prosthesis might be a practical possibility.
1962
"I see a flash!" - Button (1958, p.54)
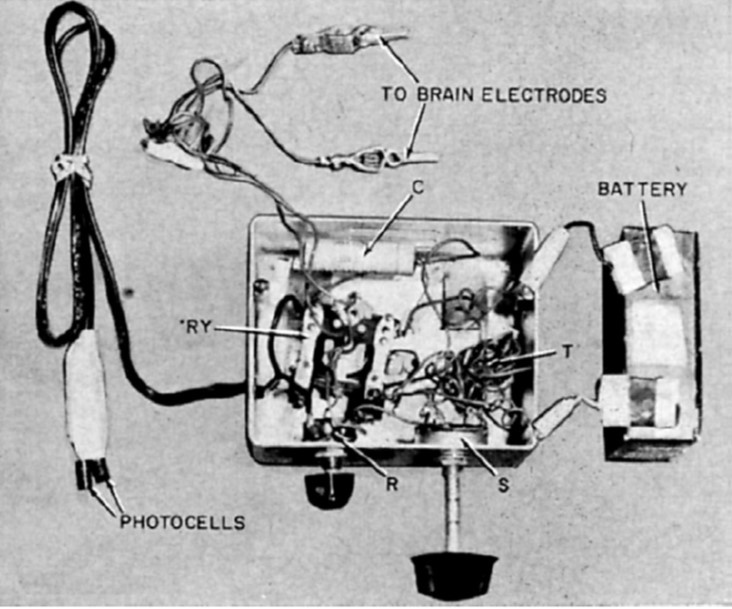
John C. Button and Tracey Putnam at Cedars of Lebanon Hospital (now Cedars-Sinai) in Los Angeles created and implanted the first ever bionic vision device in a 36-year old woman who had been completely blind for 18 years. Two pairs of stainless steel wires were implanted into the cortex and connected to a simple stimulator. The patient reported seeing vivid flashes of light and (with the use of a photocell) was able to detect the presence and relative brightness of a light source [3].
Fig. 2: Button's stimulating apparatus was connected to an array of four stainless steel wires implanted in the occipital cortex of a blind woman. (reproduced under CC BY-NC-ND 4.0 from [2])
1965
Giles Brindley, a physiologist at the University of Cambridge, had been undertaking a programme of research to support the development of a fully implanted, multielectrode wireless visual cortex stimulation device developed a fully implanted, multielectrode, wireless visual cortex stimulation device with the goal of allowing prosthesis recipients to read printed or handwritten text [5].
1966
The first Conference on Visual Prosthesis was held in Endicott House at the Massachusetts Institute of Technology. The conference chair Theodore Sterling explained that after initial difficulty in bringing together potential participants, the conference was finally held under the proviso that the proceedings would not be made publicly accessible [2].
1968
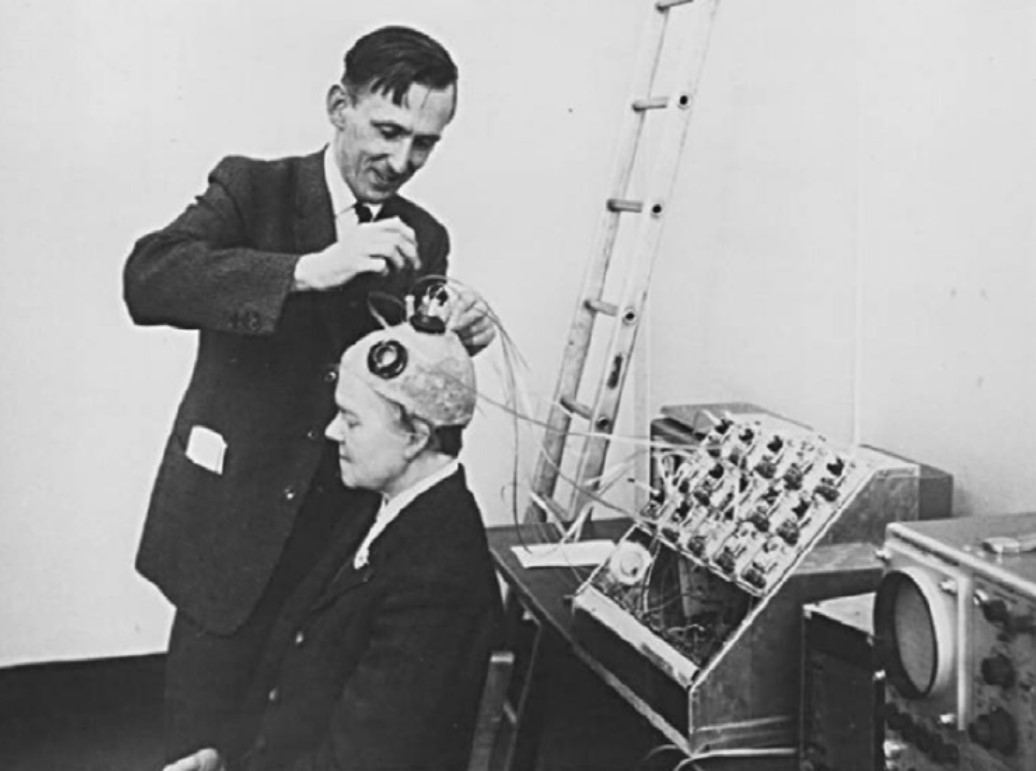
Brindley and Lewin implanted their first cortical implant that consisted of an array of 80 small, square, platinum electrodes embedded in a silicone cap. Each electrode was hard-wired to a receiving coil that was part of an array of coils also embedded in silicone and implanted under the scalp. 39 electrodes in the implant produced phosphenes across the patient’s visual field when stimulated. Centrally evoked phosphenes were small and flickering whereas peripherally evoked phosphenes differed more in size, shape, brightness, and sharpness [12].
Fig. 3: Brindley stimulating the visual cortex of his first implant recipient, placing a stimulating coil over a matched receiving coil implanted under her scalp. (reproduced under CC BY-NC-ND 4.0 from [2])
The Era of Government-Funded Research
The same year that Brindley & Lewin published their groundbreaking paper, the UK Medical Research Council formed the Neurological Prosthesis Unit, with Brindley as its director. In the US, the University of Utah instigated a sensory prosthesis research program with William Dobelle as director. In addition, the National Institute of Health (NIH) established a neuroprosthetic program at the US National Institute of Neurological Diseases and Blindness (NINDB, now known as NINDS). Thus began the era of government-funded research towards the development of a cortical visual prosthesis:
1969
The second Conference on Visual Prosthesis was held at the University of Chicago, aimed at trying to find future directions necessary to explore a possible visual prosthesis [2].
1974
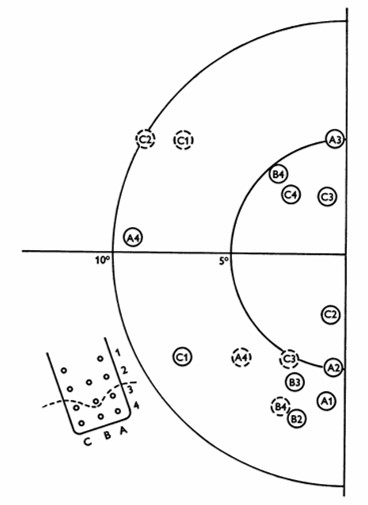
William Dobelle and his group developed a removable multielectrode cortical surface array, which was tested on 36 sighted patients undergoing occipital surgery [13] and 2 blind volunteers who had been blind for 7 and 28 years, respectively [14]. The array contained 64 hexagonally arranged platinum discs placed only over the mesial occipital cortex, each of 1-mm2 surface area. Their results in blind subject closely agreed with Brindley & Lewin's [12], noting that phosphenes could be elicited with stimulation of V2 and persisted with suprathreshold stimulation. The same was not true in sighted volunteers, where phosphenes did not always flicker and were occasionally coloured, always disappeared immediately upon cessation of stimulus, and gradually faded with continuous pulse trains [13].
Fig. 4: Phosphene map in the visual field for case no. 36. Phosphenes indicated by dashed circles appear only at high amplitudes. The electrode array and numbering system are also shown, along with a dashed line showing the postulated position of the calcarine fissure. (reproduced with permission from [14]).
1974
Over the same period, researchers from the Huntington Institute of Applied Medical Research also developed and tested a cortical array comprising smaller, "strip" arrays of 18 platinum surface electrodes, placed separately on both the occipital pole and mesial occipital cortex of normally sighted patients [2, 15]. The implant was able to elicit phosphenes, but could also cause seizures, which led the NIH to disbandon the group's effort in favor of research on "safe and effective methods for neural stimulation" [2].
1975
Another group funded by the NIH and based at the Massachusetts General Hospital in Boston had been undertaking intraoperative visual cortex stimulations on five patients throughout the early 1970s. However, only three patients reported seeing phosphenes, with the two failures attributed to postoperative swelling and patient drowsiness [2].
1978
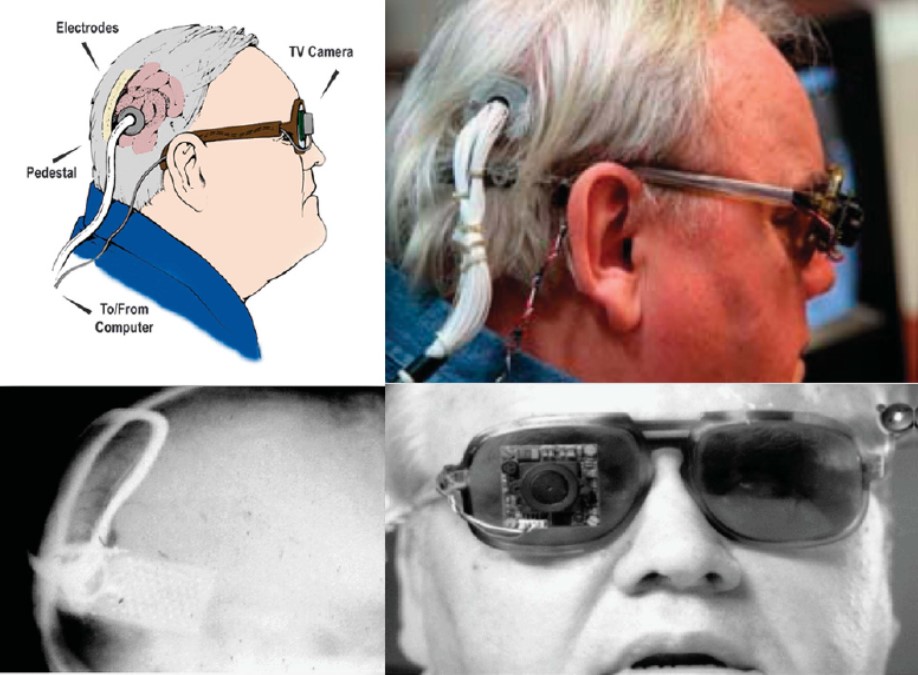
Dobelle and colleagues continued to improve their implant design throughout the 1970s, adding a transcutaneous connector to permit chronic implantation of electrodes and spectacle-mounted cameras. One of Dobelle's early patients retained his implant for over 20 years, with no reported complications of infection or seizure [2].
Fig. 5: Different views of a recipient of the Dobelle implant, first implanted in 1978. In 2000, his external hardware & software was upgraded, including the miniature camera shown, greatly enhancing the functionality of his prosthesis. (reproduced with permission from [6])
1982
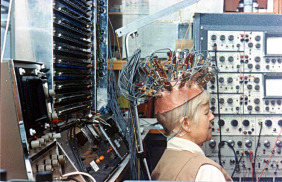
Determined to pursue the goal of reading conventional letterforms, Brindley's group continued developing improved implants into the 1980s including one with 151 electrodes [13], which unfortunately became infected and had to be removed [14].
Fig. 6: "Miss Bonnett", recipient of the last visual cortex implant built by the MRC Neurological Prostheses Unit. (reproduced under CC BY-NC-ND 4.0 from [2])
The Era of Retinal Implants
The considerable risk associated with cortical prostheses led to the scientific community to explore other locations along the visual pathway capable of producing phosphenes, which led to a new era focused on the development of retinal prostheses.
1996
Mark Humayun and colleagues showed that local electrical stimulation of the retinal surface in patients with outer retinal diseases resulted in focal light perception, even though the patients had been blind for many years [16].
2011
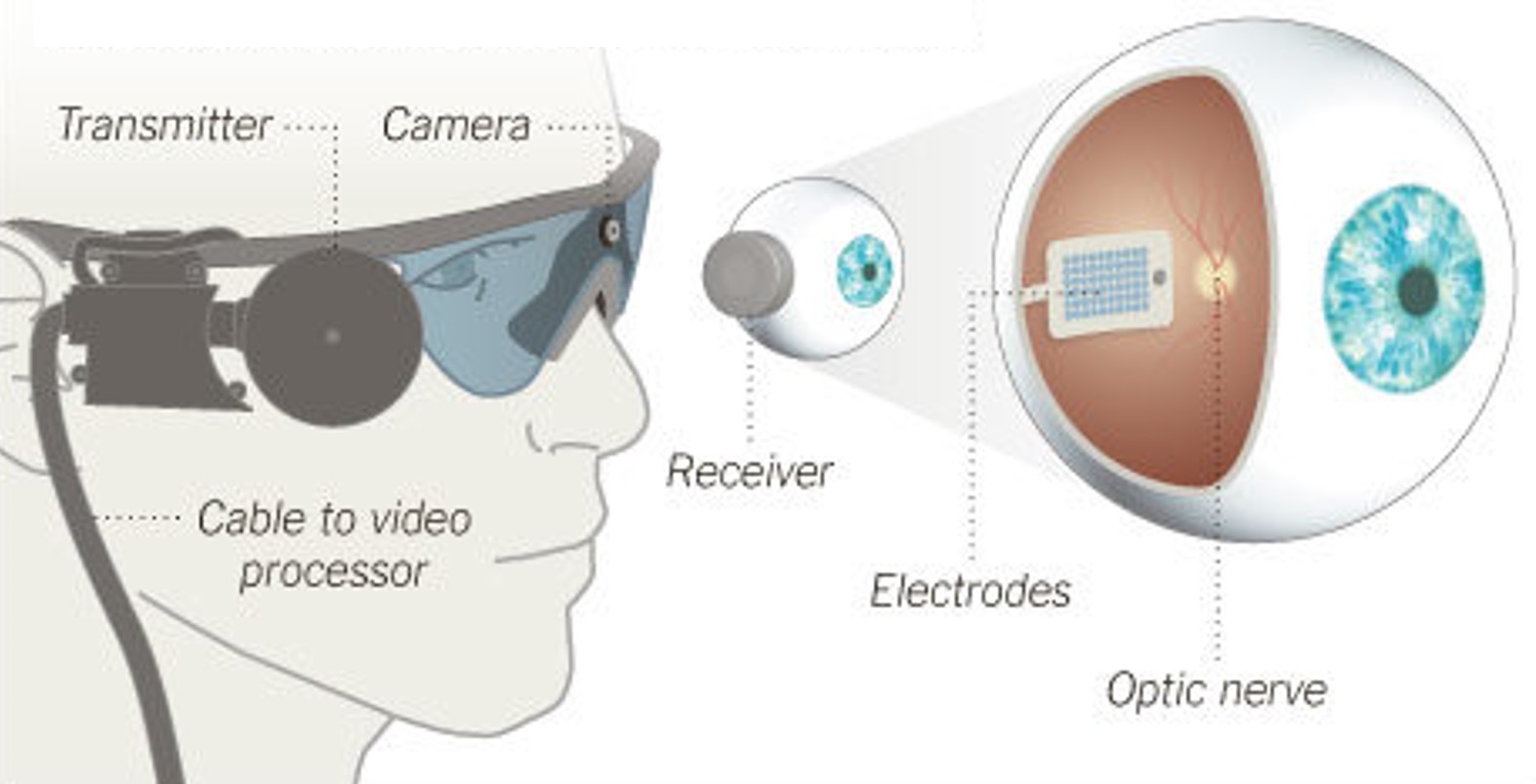
Argus II, an
epiretinal implant developed by Second Sight Medical Products, received approval for commercial use in the European Union (CE Mark). The implant was initially available at a limited number of clinics in Switzerland, France, and the United Kingdom, at an EU market price of $115,000 [17].Fig. 7: Argus II Retinal Prosthesis System (Source: Second Sight). Camera images are processed and transferred to electrodes implanted in the back of the eye.
2013

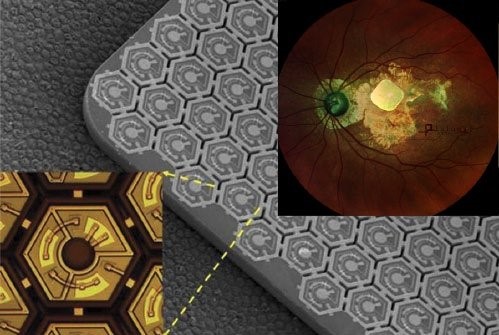
Alpha IMS, a
subretinal implant developed by Retina Implant AG, received the CE Mark as well [18]. The implant was initially available at around $130,000.Meanwhile in the US, Argus II became the first retinal implant to receive FDA approval [19].
Fig. 8: Alpha IMS, a subretinal implant consisting of a 3x3mm2 microchip with 1,500 electrodes (Source: Retina Implant AG).
2019
Retina Implant AG discontinued business activities quoting innovation-hostile climate of Europe's rigid regulatory and unsatisfactory results in patients.
2020
Stricken by fundraising issues due to COVID-19, Second Sight announced massive lay-offs and an intent to wind down operations. The company would later attempt a (failed) business merger with Pixium Vision but ultimately rebound after raising ~$60M in a public offering.
2021
Pixium Vision implanted their first of an expected 38
AMD patients with PRIMA, a subretinal implant, as part of their PRIMAvera clinical trial. This study is the last step before seeking market approval for dry AMD in Europe.In addition, Pixium expects to report 36-month data from the French feasibility study by early 2022, and is continuing clinical development in the US.
Fig. 9: PRIMA, a subretinal implant consisting of ~400 photovoltaic cells (Source: Pixium Vision).
The Future
2020s - ?
With dozens of next-generation implants in development that include retinal, optic nerve, and cortical approaches, a wide variety of bionic eye technologies should be available within a decade.
 All our content (unless otherwise noted) is licensed under
All our content (unless otherwise noted) is licensed under